how are ionic bonds formed
Ionic bonds are formed when a valence electron in the outermost shell of one of the atoms separates completely and moves into the outermost shell of another atom. Ionic bonds form when an anion bonds with a cation.
 |
| Day 8 Ionic Bonds Ct D Science Ppt Download |
Which of these is an example of an ionic bond.
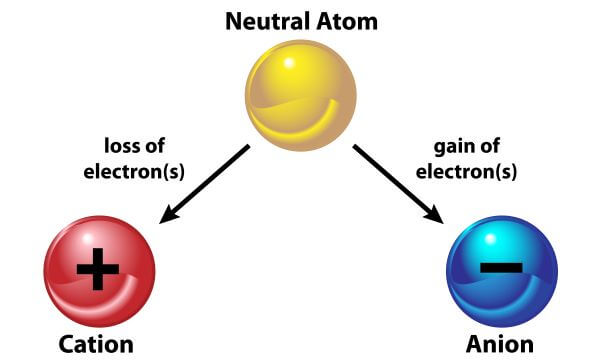
. The oppositely charged ions are strongly attracted to each other forming. Forming ionic bonds Positive and negative ions form when a metal reacts with a non-metal by transferring electrons. An ionic or electrovalent bond is formed between two ions of opposite charges. The bond is formed when an atom typically a metal loses an electron or electrons and.
Ionic Bonds In an ionic bond one atom essentially donates an electron to stabilize the other atom. Ionic bonds are basically formed when one element needs to give up its electron s to achieve an octet or chemical stability and another element needs those electron s to get stability. The ionic bond is formed. In ionic bonds the metal loses.
A basic description of the transfer of an electron between sodium and chlorine forming the sodium chloride ionic compound. What way do ionic bonds form so that the outer most energy level of atoms are filled Electron negative electrical charge proton positive electrical charge when atoms gain or lose electrons. The bond forms when the atom with less than 4 valence electrons Sodium gives its electrons to the atom with more than 4 valence. Ionic bonding is a form of chemical bonding that includes the electrostatic attraction of oppositely charged ions or two atoms with drastically differing electronegativity and it is the.
It is a type of chemical bond that generates two oppositely charged ions. An ionic bond can be formed after two or more atoms loss or gain electrons to form an ion. The compound formed is called an ionic compound. In other words the electron spends most of its time close to the bonded atom.
The loss or gain of valence electrons allows ions to. An ionic bond is formed when a metal combines with a non-metal to produce a compound. Ionic bonds are formed through the exchange of valence electrons between atoms typically a metal and a nonmetal. Learn how to write the chemical formulas for some common ionic compounds including Sodium Chloride Alumi.
During the formation of an ionic bond one of the reacting elements should form a positively charged ion. This video tells how ionic bonds form in a chemical reaction showing electron transfer from metal to non-metal and resulting charge formation resulting in i. When two or more oppositely charged ions are held together due to the presence of electrostatic force the resulting bond is termed an ionic bond. Ionic Bond Definition.
Ionic bonds involve a cation and an anion. Ionic bonding is the complete transfer of valence electron s between atoms. Ionic bonds occur between metals losing electrons and nonmetals gaining electrons. Ionic bonds connect a metal and nonmetal element.
 |
| Chemistry Unit Ionic Compounds Formation Formulae Naming Tpt |
 |
| What Are Ionic Compounds Definition Structure Properties Examples With Videos Of Ionic Compounds Ionic Character |
 |
| Chapter 7 Ionic Bonding Ppt Download |
 |
| Ionic Bond Images Browse 868 Stock Photos Vectors And Video Adobe Stock |
 |
| The Nature Of Ionic Bonding A Level Chemistry Youtube |
Posting Komentar untuk "how are ionic bonds formed"